
Press Release
03/01/2021
menu

Nevro is a medical device company and creator of HF10®,
an innovative, evidence-based treatment for chronic pain.
The company, based in Redwood City, California has a simple goal of helping more patients suffering from chronic pain achieve lasting relief.

While traditional spinal cord stimulation (SCS) has been around for over 30 years, HF10 created a disruption by offering a next-generation approach that provides patients with substantially better long-term outcomes.

HF10 has been available in Europe and Australia since 2011 and received FDA approval in the United States in 2015. Uniquely, the FDA designated HF10 as superior to traditional SCS for the treatment of back and leg pain.
Worldwide, tens of thousands of patients are enjoying more freedom and improvement in their daily lives with HF10.
HFX is approved for the treatment of chronic intractable pain of the trunk and/or limbs. Only a physician can recommend a specific treatment option.
(Select all that apply)
No Pain
0 1 2 3 4 5 6 7 8 9 10Worst Pain Imaginable
No Pain
Worst Pain Imaginable
(Select all that apply)
(Select all that apply)
At this time, it looks like you might not be an appropriate candidate for HFX. The next best step is to talk with the physician who is treating your pain about your treatment options.
FIND A PHYSICIANPain score is:
8/10
Struggling with chronic pain for:
Over 3 months
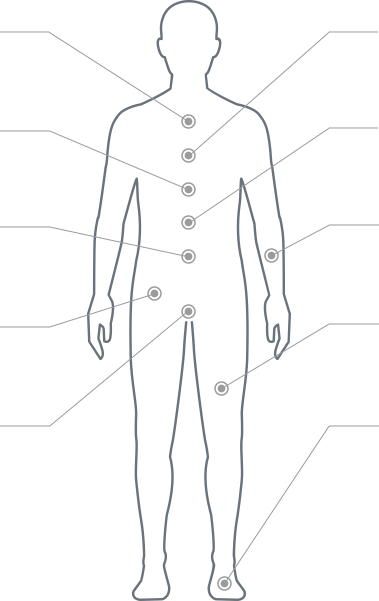
Pain is located in:
Legs, Buttocks, Arms
I’ve already tried:
Epidural Injections, Back Surgery, Nerve Ablations
Please use my information to provide me with phone, email, or text message communications about HFX and other health information. I understand these communications may include advertisements for goods and I can unsubscribe at any time.
All information will be used in accordance with Nevro’s Privacy Policy.
Your results and discussion guide will be on their way shortly.
Please find a copy at the email address provided.
We did not update your responses. Your results and discussion guide will be based on your previous responses. Please find a copy at the email address provided.
Please be aware that the website you have requested is intended for the residents of a particular country or region, as noted on that site. As a result, the site may contain information on medical devices and other products or uses of those products that are not approved in other countries or regions.
Do you wish to continue?
Please be aware that the website you have requested is intended for the residents of a particular country or region, as noted on that site. As a result, the site may contain information on medical devices and other products or uses of those products that are not approved in your country or region.